Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Young Healthy Man with Mixed Aortic and Mitral Valve Disease and Aneurysm of Sinus of Valsalva Causing Acute Antero-Lateral Myocardial Infarction, Cardiogenic Shock and Left Ventricular Failure: Lessons Learned
*Corresponding author:Khin Phyu Pyar, Professor and Head/Senior Consultant Physician, Department of Medicine/ Department of Nephrology, Defence Services Medical Academy, Defence Services General Hospital (1000-Bedded), Yangon, Myanmar.
Received: March 12, 2024; Published: March 15, 2024
DOI: 10.34297/AJBSR.2024.21.002898
Case Summary
young healthy man presented with dyspnoea on exertion for 3 weeks. Physical examination was compatible with mixed aortic and mitral valve disease; echocardiogram revealed aneurysm of sinus of valsalva (SOVA) with mitral regurgitation and aortic regurgitation. While waiting CT coronary angiogram and surgery, he had one episode of torrential chest pain, extensive myocardial infarction, left ventricular failure with cardiogenic shock. Thrombolytic therapy and inotropes could not save the patient. We report autopsy findings and review the current literature.
Keywords: Myocardial infarction, Left ventricular failure, Cardiogenic shock, Aneurysm of sinus of valsalva, Mixed aortic and mitral valve disease
Introduction
A Sinus of Valsalva Aneurysm (SOVA) is an abnormal dilatation of the aortic root located between the aortic valve annulus and the sinotubular junction. Embryologically, the SOVA forms first as a blind diverticulum secondary to pressure forces on the aortic root. Thus, congenital defects potentiating these pressure forces can lead to development of a SOVA. It occurs as a consequence of weakness of the elastic lamina at the junction of the aortic media and the annulus fibrosis. The function of the normal sinuses is to prevent occlusion of the coronary artery ostia during systole when the aortic valve opens. The normal sinus diameter is less than 4.0cm for men and 3.6cm for women. SOVA is a rare condition; 0.09% of the general population is affected [1]. It can be either congenital or acquired. It often presents as an incidental finding during cardiac imaging [1]. The presentation may range from silent asymptomatic cases detected incidentally to catastrophic rupture [2]. The presenting symptoms depend on their mass effect on the coronary arteries, heart valves, and other adjacent structures. Right cusp is more affected than left [3].
There are no specific guidelines for the diagnosis and management of SOVA. Echocardiogram is the standard imaging technique for diagnosis of sinus of Valsalva aneurysm [3,4]; Cardiac Computed Tomography Angiography (CCTA) is indicated for patients with low to intermediate risk for Coronary Artery Disease (CAD) prior to surgical repair. Surgery is the treatment of choice [3]; however, transcatheter techniques is the nonsurgical alternatives for repair [2]. Repair is generally required for ruptured aneurysms; unruptured aneurysms encroaching on nearby structures, causing myocardial ischemia, or having the potential to rupture warrant repair. Surgery should be prompted in patients with symptomatic, large, or rapidly expanding unruptured SVAs, as well as those unruptured SVAs that contain intraluminal thrombi, have a mass effect on surrounding structures, or are recurrent. Surgical outcomes are generally good with favourable prognosis and minimal recurrence [5]. Reports on repair of SOVA were fairly good [4]. One-third of cases with SOVA were prone to rupture [3]. If ruptured SOVA remain untreated, the prognosis is poor, with a 1‐year life expectancy [1]. Myocardial ischemia is a potentially ominous prognostic sign in patients with SOVA. The poor outcome of ruptured SOVA with conservative treatment leads us to consider the patient for emergency surgical therapy [4].
Active surgical repair of an USVA can be achieved with satisfactory results in patients combined with other cardiovascular lesions [6,7]. Elective surgical repair can be performed with low risk [3,8,9]. Mechanical arotic prosthesis is indicated if SOVA is associated with aortic regurgitation [10,11]. Performing valve-sparing by applying a remodeling technique operation completed with annuloplasty reduces aortic valve insufficiency, avoiding side-effects related to implanted valves [11]. In cases of rupture, prognosis is poor and surgical repair is always required [12].
Case Presentation
A-28-years-old man was fit and healthy till 3 weeks prior to admission. He suffered dyspnoea on exertion for 3 weeks and it was not associated with fever, cough or sputum. There was no history of rheumatic fever. He was a chronic smoker.
His blood pressure was 130/80 mmHg. Pulse rate was 80/minutes with sinus rhythm. Apex beat was displaced and heaving. Pansystolic murmur was heard over mitral area. Early diastolic murmur was heard over aortic area and left parasternal area; features pointing to mixed aortic and mitral valve disease. The etiology of regurgitant lesions was thought to be due to rheumatic fever as it was common in Myanmar.
There were no features of heart failure or infective endocarditis. Figure 1 shows initial ECG on admission. ECG revealed left axis deviation with left ventricular hypertrophy. Figure 2 demonstrates Chest Xray. Heart size was upper limit of normal and lung fields were clear.

Figure 1: Assessment of the effect of emergency radiation therapy on patients, an effect on overall survival (OS).
Figure 3 is echocardiogram. It demonstrated a ring-shaped mass attached to aortic valve and its convexity towards left ventricle. Left ventricle was dilated with features of moderate mitral regurgitation and aortic regurgitation. As we did not see the ring-shaped mass attached to aortic valve, we traced the similar photo in internet. It was similar appearance with aneurysm of sinus of Valsalva.
He was treated with anti-failure treatment with diuretics, beta-blocker and ACEI. We discussed with cardiac surgeon and planned for CT coronary angiogram and possible surgery. Blood investigations were normal except normochromic normocytic anemia; hemoglobin was 10.5gm%; total WBC was 8.4X109/L; platelet count was 299X109/L; blood urea was normal (37.9mmol/L; serum creatinine was 1.1 mg%; cholesterol was 161mg%; triglyceride was 92mg%; and, uric acid was 7.1mg%. Serology for retroviral infection was negative. CT coronary angiogram was pending. He developed sudden onset of severe excruciating chest pain at midnight while he was on waiting list for surgery. He was desperate; very dyspnoeic with sweating; low blood pressure of 80/50mmHg; tachycardiac of 120/minutes; left ventricular failure. Event ECG is shown in Figure 4. Event ECG revealed ST depression at II, III, AVF, V4-6, sinus tachycardia, T inversion at I & AVL, tall T at V2-3; features were suggestive of extensive antero-septal infarction with lateral extension. Bedside echocardiogram demonstrated that LVEF was 45%; dilated left ventricle; moderate mitral regurgitation and aortic regurgitation; aneurysm at sinus of Valsava; and no pericardial effusion. Therefore, he was treated as a case of acute coronary syndrome with streptokinase, inotropes, morphine, non-invasive ventilation and oxygen therapy. ECG 2hours after thrombolysis showed left bundle branch block, ST depression at II, III, AVF, V4-6, sinus tachycardia, Q/S inV1-3, T inversion at I & AVL. It is revealed in Figure 5. The patient expired 6hours after onset of chest pain. Post-mortem examination was done.
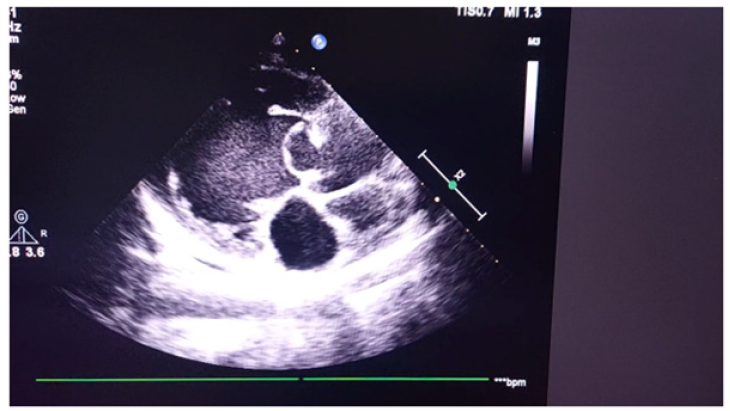
Figure 3a: Echocardiogram demonstrating a ring-shaped mass attached to aortic valve and its convexity towards left ventricle.

Figure 3b: Echocardiogram demonstrating a ring-shaped mass attached to aortic valve and its convexity towards left ventricle.
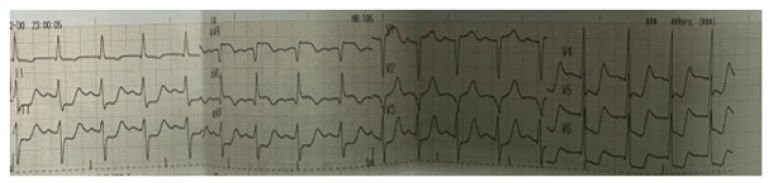
Figure 4: Event ECG showing ST depression at II, III, AVF, V4-6, sinus tachycardia, T inversion at I & AVL, tall T at V2-3.

Figure 5: ECG after thrombolysis showing left bundle branch block, ST depression at II, III, AVF, V4-6, sinus tachycardia, Q/S inV1-3, T inversion at I & AVL.
Figures 6-7 are post-mortem examination of anterior and lateral surface heart. They revealed hyperemia of whole surface of left ventricle indicating very extensive myocardial infarction.

Figure 6: Post-mortem examination of anterior surface heart showing hyperemia over surface of left ventricle.
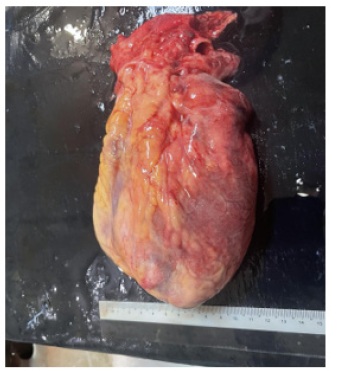
Figure 7: Post-mortem examination of lateral view heart showing hyperemia over surface of left ventricle.
Figure 8 is post-mortem examination of cut section of left ventricle at aortic valve. It showed both right and left coronary ostia just above 3 cusps of aortic valve were normal; they were not occluded with clots.
Figure 9a-9b are post-mortem examination of cut section of left ventricle. The forceps pointed the aneurysm of Sinus of Valsalva; it was attached to posterior cusp of aortic valve. And it was close to left coronary ostia. The myocardium of the whole left ventricular was hyperemic indicating very extensive myocardial infarction.
Figures 10a-10b show the size of aneurysm. The aneurysm of Sinus of Valsalva was filled with water to measure the size; it was 4.2 X 5.6 cm in diameter. It was located next to left coronary ostia (Figures 11-13).
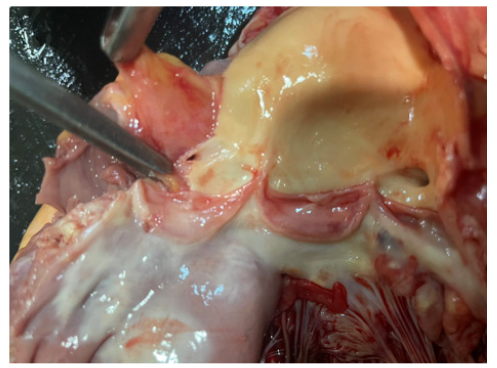
Figure 8: Post-mortem examination of cut section showing intact both right and left coronary ostia just above 3 cusps of aortic valve.
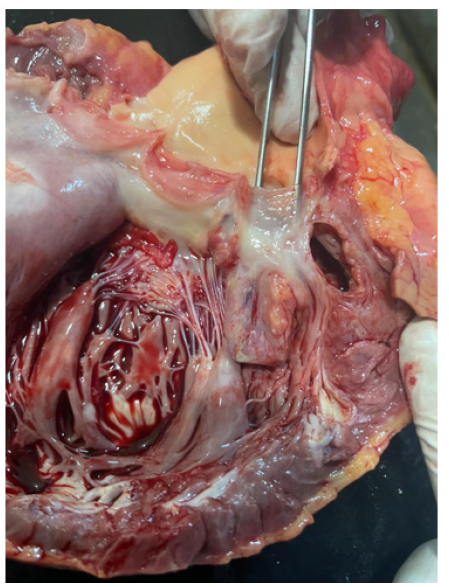
Figure 9a: Post-mortem exasmination of cut section of left ventricle and the forceps pointing the aneurysm of Sinus of Valsalva attached to posterior cusp of aortic valve. The myocardium of left ventricular is hyperemic.
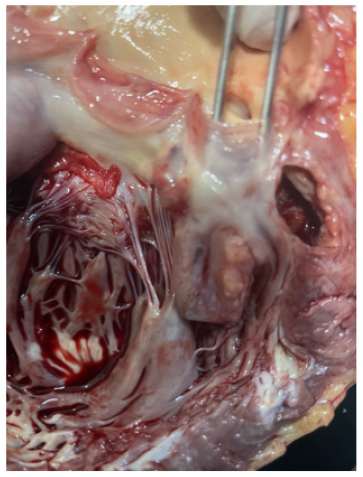
Figure 9b: Post-mortem examination of cut section of left ventricle and the forceps pointing the aneurysm of Sinus of Valsalva attached to posterior cusp of aortic valve. The myocardium of left ventricular is hyperemic.
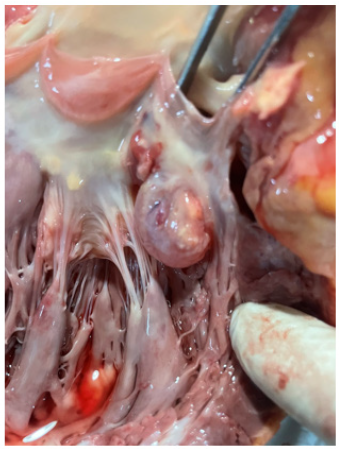
Figure 10a: The aneurysm of Sinus of Valsalva was filled with water to measure the size revealing 3.2 X 3.6 cm in diameter. It was located next to left coronary ostia.
Discussion
This patient was found to have mitral regurgitation and aortic regurgitation. The likely causes of chest pain in patient with aortic valve disease were coronary osteitis, coronary obstruction in hypertrophied left ventricle during ventricular systole, imbalance between coronary supply and hypertrophied left ventricle, tachycardia. In this patient, SOVA caused obstruction of left coronary ostia and produced chest pain. His initial ECG showed T inversion in V4-5. It supported the case report ‘cases with atypical chest pain caused by a giant unruptured aneurysm of the right sinus of Valsalva SOVA’, described by Serban [13]. The function of Sinus of Valsalva is to preserve coronary filling during ventricular systole. In case of the aneurysm of Sinus of Valsalva, it cannot maintain the opening of coronary ostia during ventricular contraction; therefore, it leads to coronary occlusion and myocardial infarction. If it obstructs right coronary ostia, right coronary artery will be involved. If it obstructs left coronary ostia, left coronary artery will be involved. In this patient, the SOVA was situated next to left coronary ostia; hence, it caused occlusion of left coronary artery. It caused total obstruction of left anterior descending artery and extensive antero-lateral infarction; cardiogenic shock, acute left ventricular failure and death. SOVA involving left cusp was mentioned by Sarkar, et al.; it produced myocardial infarction [14].
Backward flow of blood from aorta due to aortic regurgitation during ventricular systole may cause more filling of blood into SOVA; therefore, larger volume blood in the SOVA aggravated occlusive effect to left coronary ostia. Moreover, volume overload due to regurgitant effect of mitral regurgitation and aortic regurgitation also produced advancement in left ventricle hypertrophy; imbalance between coronary supply and demand. If we had done CTCA and early resection of SOVA and aortic valvuloplasty, we would have saved this patient. In this patient, he might need repair of mitral valve too. In poor resource setting, CT scan may not be easily available; moreover, prosthetic valves of various sizes may not be easily available. Having anterolateral myocardial infarction in event ECG was suggestive of involvement of left coronary artery in this patient. Moreover, development of LBBB in serial ECG indicated that left bundle branch of conducting system was affected. In post-mortem examination, SOVA was arising from left cusp of aortic valve and it was situated next to left coronary ostia. This case gave good clinic-pathological correlation. This is another reason for case reporting.
Despite complete aortic root and valve replacement being considered the safest approach to large SVAs complicated with aortic insufficiency, valve-sparing procedures should not be overlooked in case of a dilated aortic root with uncalcified aortic valve. Performing valve-sparing by applying a remodeling technique operation completed with annuloplasty reduces aortic valve insufficiency, avoiding side-effects related to implanted valves [11,10]. According to Feldman & Roman, SOVA commonly involve the right or noncoronary sinuses. The predominant fistula was mentioned as the right sinus of Valsalva to the right ventricle [3,15]. It is one reason for case reporting; SOVA involving left sinus of Valsalva. They also pointed out that men are more affected with male to female ratio of 4:1. Although a reported incidence of SOVA in Asian population was higher [16], this case was our first experience.
Though Marfan's syndrome, Ehlers‐Danlos syndrome, or other connective tissue disorders are frequently associated with congenital SOVA, this patient did not have features of them. Acquired SOVAs are similarly associated with connective tissue pathologies. Infectious etiologies are well established mechanisms for elastic tissue weakening. Syphilis, bacterial endocarditis, and tuberculosis have each been linked to SOVA formation. This patient did not have features of infective endocarditis; serological test for syphilis done one year ago was negative. Though he was a chronic smoker, there was no evidence of atherosclerosis in cut section of aorta. Having regurgitant lesion of both mitral and aortic valves in this patient was highly suggestive of rheumatic etiology. SOVA associated with aortic valve abnormality was uncommonly reported [17]. Previous reports on SOVA rarely mention about association with mitral valve disease. This is one reason for sharing experience.
Moreover, patients with a Biscuspid Aortic Valve (BAV) were more likely to develop SOVA, all 3 valves of aortic valves were intact in this patient. The association with aortic regurgitation was recorded in nearly half of patients with SOVA in one study [3]; however, this patient had both mitral and aortic regurgitation. Being rare report on association with mitral valve disease is another reason for case reporting.
This patient presented with dyspnoea and he developed severe LVF with cardiogenic shock following acute
myocardial infarction. ‘A 68-year-old woman with a giant aneurysm of the sinus of Valsalva of the non-coronary aortic cusp leading to heart failure, diagnosed with transthoracic echocardiography’ was reported by Wierda, et al. [18,19]. In this patient, the actual size of SOVA in autopsy was difficult to measure as it was collapsed. There was no thromboses inside the aneurysm. It was nearly 5cm after filling with water into SOVA; there was no evidence of rupture. As the site of SOVA in this patient was very close to left coronary sinus and all clinical features as well as ECG changes pointing to occlusion of left coronary artery (left anterior descending), the cause of death was attributed by large SOVA obstructing left coronary sinus. A massive aneurysm (SOVA) (in excess of 4 cm) involving the right coronary sinus of the aorta was reported as an accidental finding; and, the cause of death was due to fatal arrhythmia [20]. They also mentioned that the autopsy images on SOVA was very uncommon. This is another reason for sharing images and experiences. In cases of rupture, prognosis is poor and surgical repair is always required [12,21].
Conclusion
Causes of non-coronary angina like the aneurysm of Sinus of Valsalva (SOVA) should be considered in young patient with acute myocardial infarction. SOVA has peculiar echocardiographic features. SOVA is a very rare congenital cardiac anomaly with variable clinical presentation ranging from asymptomatic detection on imaging to acute coronary syndrome, left ventricular failure and sudden cardiac death.
Ethical Approval
Our institution does not require ethical approval for reporting cases.
Funding
The authors received no financial support for publication of this article.
Informed Consent
The informed consent for publication in this article was obtained from patient.
Acknowledgements
The authors would like to thank the patient and family for giving consent to this article. Also, to all doctors and nursing team for making great efforts in caring him. The authors acknowledged the following team; Professor Ni Ni Win and cardiology team, Dr Kyaw Thura and cardiac surgical team, Professor Thet Naing, Professor Myint Zaw, Professor Kyaw Zay Ya and Professor Ko Ko Lwin for administrative support.
Declaration of Conflict of Interest
The authors declared no potential conflicts of interests with respect to authorship and publication of this article.
References
- Weinreich M, Yu PJ, Trost B (2015) Sinus of valsalva aneurysms: Review of the literature and an update on management. Clin Cardiol 38(3): 185-189.
- Shaw M, Sharma A, Kumar S (2020) Sinus of Valsalva Aneurysms: Basic Concepts and Imaging Evaluation Using Multidetector Computed Tomography. J Thorac Imaging 35(1): w30-w38.
- Moustafa S, Mookadam F, Cooper L, Adam G, Zehr K, et al. (2007) Sinus of Valsalva aneurysms-47 years of a single center experience and systematic overview of published reports. Am J Cardiol 99(8): 1159-1164.
- Vural KM, Şener E, Taşdemir O, Bayazıt K (2001) Approach to sinus of Valsalva aneurysms: A review of 53 cases. Eur J Cardiothorac Surg 20(1): 71-76.
- Nguyen Q, Vervoort D, Phan K, Luc JGY (2021) Surgical management for unruptured sinus of Valsalva aneurysms: A narrative review of the literature. J Thorac Dis 13(3): 1833-1850.
- Qiu J, Xie E, Wang Y, Wang W, Yu C, Luo X (n.d.).
- Kulkarni M, Rahiman S, Samal A, Mishra B, Vijayalakshmi I, et al. (2008) Aneurysms of Sinuses of Valsalva 170.
- Wingo M, de Angelis P, Worku BM, Leonard JR, Khan FM, et al. (2019) Sinus of Valsalva aneurysm repairs: Operative technique and lessons learned. J Card Surg 34(6): 400-403.
- Diwakar A, Patnaik SS, Hiremath CS, Chalam KS, Dash P (2019) Rupture of sinus of Valsalva-A 15 years single institutional retrospective review: Preoperative heart failure has an impact on post operative outcome? Ann Card Anaesth 22(1): 24-29.
- Stróżyk A, Kołaczkowska M, Fijałkowska J, Siondalski P, Fijałkowski M (2018) Sinus of Valsalva rupture in a patient with a mechanical aortic prosthesis: Aneurysm dissecting into the interventricular septum. Kardiol Pol 76(12): 1742.
- Pólos M, Șulea CM, Benke K, Ágg B, Kovács A, et al. (2020) Giant unruptured sinus of Valsalva aneurysm successfully managed with valve-sparing procedure-a case report. J Cardiothorac Surg 15(1): 6.
- Doost A, Craig JA, Soh SY (2020) Acute rupture of a sinus of Valsalva aneurysm into the right atrium: A case report and a narrative review. BMC Cardiovasc Disord 20(1): 84.
- Serban AM, Bătrâna N, Cocoi M, Ianoș R, Moț Ștefan, et al. (2019) The role of echocardiography in the diagnosis and management of a giant unruptured sinus of Valsalva aneurysm. Med Ultrason 21(2): 194-196.
- Sarkar M, Wehman B, Mukherjee R, Taylor BS (2019) Left sinus of Valsalva aneurysm presenting as myocardial ischemia. J Thorac Cardiovasc Surg 157(3): e167-e168.
- Ramalingam Vadivelu, Manoj Kumar Rohit, Mukesh Yadav (2013) Ruptured sinus of Valsalva aneurysm from left coronary sinus into right atrium: A rare anomaly with an odd presentation. BMJ Case Rep bcr2012007855.
- Feldman DN, Roman MJ (2006) Aneurysms of the sinuses of Valsalva. Cardiology 106(2): 73-81.
- Urbanski PP, Hirao S, Irimie V (2020) Root repair in patient with huge sinus Valsalva aneurysm and severe aortic regurgitation. Gen Thorac Cardiovasc Surg 68(5): 530-533.
- Wierda E, Koolbergen DR, de Mol BAJM, Bouma BJ (2018) Rupture of a giant aneurysm of the sinus of Valsalva leading to acute heart failure: A case report demonstrating the excellence of echocardiography. Eur Heart Journal Case Rep 2(3): yty090.
- Chan N, Charalambous M, Fuschetto DP, Fuschetto O, Makaryus JN (2020) Severe compression of the left circumflex coronary artery by a large sinus of Valsalva aneurysm. J Cardiovasc Comput Tomogr 14(5): e26-e28.
- Farì G, Pennacchia I, Stigliano E, Oliva A, Carbone A, et al. (2020) Right sinus of Valsalva aneurysm. Cardiovasc Pathol 47:107209.
- Chen J, Liang HN, Wu L, Dong SH, Li JH (2019) Right sinus of Valsalva aneurysm spontaneously dissecting into the interventricular septum in a rare case of Behcet’s disease. Eur Heart J Cardiovasc Imaging 20(5).







 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.